|
Michael Bluejay’s
|
Contact • MBJ's home page |
Disposable
Batteries explained and compared
|
|||||||||||||||||||||||||||||
| What
battery to use instead of alkaline |
|||
| NiMH |
LSD NiMH |
NiZn |
|
| You're using a high drain
device |
|
|
|
| You go through batteries
quickly |
|
|
|
| You need a lot of voltage |
|
||
| You need long shelf life |
|
||
| Most other applications |
|
|
|
Here's why:
- High-drain devices. Alkalines aren't good for high-drain devices because they can't supply the juice fast enough. So they have short run-time, if they work at all.
- Going through batteries quickly. If that's the case, you need a rechargeable battery.
- Need high voltage. NiZn is probably what you need.
- Long shelf life. Other batteries offer decent
shelf life, and can either be recharged or last longer, and
aren't nearly as susceptible to leaking. For smoke
detectors, Lithiums are best, since they can last 7 years.
Where to buy. Man, if you can't find alkaline
batteries, you've got bigger problems. :)
High-drain performance. Standard alkalines don't work well (or at all) in high-drain devices like digital cameras, because they can't pump out the juice fast enough. There are special high-drain versions like Duracell Ultra, Energizer Advanced Formula, and Kodak Photolife which do work, but high-drain devices go through batteries quickly no matter what kind of battery, so the better solution for high-drain devices is to use rechargeables so you're not always buying batteries.
Capacity. Alkalines have decent capacity, though
the capacity is rarely stated on the packaging. That's not
too much of a problem, because there's not much difference in
capacity from brand to brand (as long as you're comparing
standard to standard, and high drain to high drain). They're all
pretty much the same, despite the manufacturers' commercials.
Consumer Reports found that the spread between the best and
worst alkalines was only 9-15%. Tests
by
ZBattery also showed little difference between name-brand
alkalines. Someone on a web forum said that it's possibly
to squeeze more life out of seemingly dead alkalines by tapping
them against a hard surface, but I have yet to test that theory.
Incidentally, refrigerating alkalines doesn't make them keep longer, which makes sense, since they last for years on their own anyway.
Voltage. The initial voltage is 1.5V, which is just about perfect for a fresh battery. The lower-voltage NiMH's (1.2V) might not be powerful enough for some applications. (more) However, as you use an alkaline, its voltage drops faster than a NiMH would, so you can often get more runtime out of a NiMH. So this means that which battery gives more runtime in your device depends on your device. For some devices it'll be alkalines, for some it'll be NiMH. (Though in general, for various reasons, I prefer NiMH to alkaline.)
When your alkalines are too dead to power higher-drain devices, they'll have a second life in remote controls and possibly clocks, which need only a little voltage to run. Also, you can make a battery pack with a battery holder from Radio Shack or Amazon to milk your alkalines completely. For example, I have some battery-powered Christmas lights that expect 3 x AA, or 4.5V. I have some alkaline AA's that are around 1.15V. (I don't buy alkalines, I salvaged these from elsewhere.) Three of them would be only 3 x 1.15V = 3.45V, which would be kind of weak for the lights. But putting four of them in a battery pack gives me 4 x 1.15V = 4.6V, which is just about perfect.
Charging & Cycle Life. There are a few battery chargers that purport to charge standard alkalines. This is pretty much a worthless gimmick. First off, the batteries can be charged only a few times if you've run them down all the way (which is how most people use batteries). And the newer high-drain alkalines are especially resistant to recharging. Next, the batteries are more prone to leaking, both during and after charging. If they leak while inside your device, they can easily ruin it. Oh, and you'll need a special charger. A standard charger won't work. (For hobbyists, Afroman offers an article about how to build your own alkaline recharger.)
Why anyone would want to go through all this is
beyond me. If you want to recharge your batteries (and I
hope you do), just get a real rechargeable, like NiMH.
If you must recharge standard alkalines, and
you've purchased the special charger for doing so, then you'll
get the most recharge cycles by keeping your alkalines "topped
off". That is, the sooner you charge them, the better.
You'll get much more total energy out of the battery over its
life that way. Also, be aware that alkalines charge much
more slowly (~8-16 hours) than real rechargeables. (TechLore)
Leaking. Alkalines leak more frequently than any other kind of battery. If you want to avoid leaks, don't use alkalines!
Three of the biggest battery-makers
offer to replace any equipment damaged by their batteries.
The Rayovac and Energizer guarantees seem to cover leakage, but
the Duracell wording is a little more ambiguous (and they
wouldn't answer my request for clarification). So if you
do use alkalines, choosing Rayovac or Energizer might afford
some measure of insurance.
- Rayovac: "Guarantee on all batteries*. We will replace or repair at our option, any device damaged by this battery if sent with batteries prepaid to the address below."
- Energizer:
"We will repair or replace, at our option, any device damaged
by these Energizer® batteries.... Contact 1-800-383-7323."
- Duracell:
"Should any device be damaged due to a battery defect, we will
repair or replace it at our option if it is sent with the
batteries, postage paid to [the address below]."
(I want to call out Duracell for special mention for having
especially pathetic customer service. I emailed them and
asked whether leaking alkalines were covered under the "damaged
due to a battery defect" clause. In response, they sent me
a generic form letter about their replacement policy which said
nothing about leaking alkalines. I wrote back, pointing
out that they didn't answer my question, and asking again
whether leaking alkalines were covered? They responded
with the SAME useless form letter! I wrote a third time,
asking them to answer my question rather than sending me useless
form letters, but they never replied. If you want a
guarantee against leaking batteries, don't buy Duracell.)
Things that increase the chances of alkaline leakage include:
- Trying to charge them
- Mixing fresh and used batteries in the same device
- Mixing alkalines and other types of batteries
- Long exposure to high temperatures
- Deep discharging
- Being dead (don't let dead batteries sit in a device)
- But sometimes alkalines seem to leak for no apparent reason
The reason why mixing fresh/used or
alkaline/other batteries together increases the chance of
leaking is that one battery will likely become exhausted first,
and it can go to very low or even negative voltage levels, and
low or negative voltage greatly increases the chances of
leakage. (Energizer,
PDF)
DEALING WITH LEAKS. Don't touch the leaked
material. If you accidentally touch it then flush the
affected area for 15 minutes with lots of water.
If an alkaline leaks, even a little bit, throw it away or recycle
it. Don't try to use it againit's just gonna
continue leaking.
If an alkaline leaked, clean up as much of the solid or liquid matter as you can with tissue paper and/or cotton swabs. After that, use cotton swabs with vinegar or lemon juice. The acid will neutralize the leaked alkaline material to prevent further corrosion. An old toothbrush or tiny wire brush might help. (If using one of those, wear safety glasses, so you don't flick the solution into your eyes.)
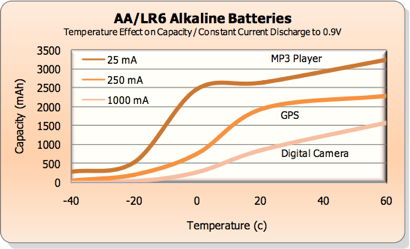 Temperature.
Alkalines
perform poorly at subfreezing temperatures (reduced capacity),
as this chart from Energizer (PDF)
shows. Nickel Zinc and
Lithium are better for
below-freezing applications. Alkalines maintain their
voltage and deliver enough current in freezing temperatures, (source)
but capacity is a problem.
Temperature.
Alkalines
perform poorly at subfreezing temperatures (reduced capacity),
as this chart from Energizer (PDF)
shows. Nickel Zinc and
Lithium are better for
below-freezing applications. Alkalines maintain their
voltage and deliver enough current in freezing temperatures, (source)
but capacity is a problem.
Recycling. Here's another reason not to use
alkalines: They're harder to recycle than rechargeable
batteries. Alkalines aren't nearly as hazardous as NiCd's,
but they do contain useful metals, and it's better for those
metals to be reclaimed by recycling rather than strip-mining
mountains. In Europe recycling is easyevery store that
sells batteries must take them back for recycling too. In
the U.S. it's tougher: while recycling for NiMH, NiZn, and
NiCd is widespread (see RBRC),
there
just aren't nearly as many places to recycle alkalines.
That's because the process just isn't as cost-effective for the
recyclers. A handful of retailers collect do collect them,
though I don't know of any who collect them at all their
nationwide stores. For most of us, that means our only
option is to mail
them to a recycling company, as well as pay a small fee to
that company. I hope retailers who read this will start
offering to collect alkalines from their customers as an extra
service, and then ship the batteries to the recyclers by
freight.
In California, all batteries are considered
hazardous materials, so they can't just be thrown in the
trash. Check with your county government about collection
facilities in your area.
Alkalines used to have a fair amount of toxic mercury, but Congress banned mercury in batteries except in trace amounts starting in 1996. (There's an exception for button batteries, the circular kind that go in watches and calculators, which can still have mercury. Radio Shack accepts those for recycling.)
More info: Energizer's alkaline FAQ (PDF), Energizer tech specs (PDF)
Lithium lots of power, but expensive, and can't be recharged
- Work great in high-drain devices
- Work well in sub-freezing temperatures
(source, PDF)
- Loooong shelf life. (9V varieties can power smoke alarms for a few years.)
- Lightweight - 1/3 the weight of alkalines (source)
- No good rechargeable version, except for 9V. Compared
to NiMH, rechargeable lithiums have less capacity, cost more,
and aren't very reliable (but rechargeable
9V lithiums are good.)
- More expensive
- High voltage can fry devices (Burned
out the bulb in PC
Magazine test.)
- Can't fly with extra batteries in checked luggage (must be
in carry-on). (DoT)
- Good luck finding a place to recycle them
- Small possibility of explosion
There's usually a better choice than lithiums. They can't be recharged (9V can, but not other sizes), and the only benefits they offer over other batteries are that they're lightweight (who cares?) and that they work in subfreezing temperatures. I can think of only one case where lithiums have a clear advantage: Smoke alarms. A 9V lithium battery in a smoke detector often lasts a few years. This is nice especially given that smoke detector batteries are bothersome to replace. And when you go through only one battery every few years, rechargeability becomes less important.
Explosion hazard. It's rare for lithium batteries to explode, but they're more likely to than other kinds of batteries. That's why you can't send them in the U.S. mail and why they can't go in your checked luggage when you fly. Here's a story about someone who nearly lost a finger when the lithium cell in his flashlight exploded.
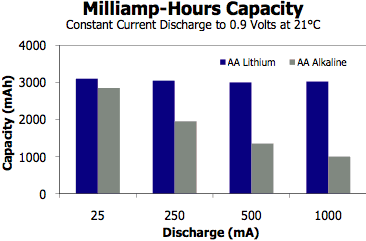 High-Drain
Performance. Lithiums work better in high-drain
devices than alkalines, as the chart at right shows. (Energizer,
PDF)
(But
then again, so do NiMH, NiZn, and high-drain Alkalines, without
most of the downsides of lithium.)
High-Drain
Performance. Lithiums work better in high-drain
devices than alkalines, as the chart at right shows. (Energizer,
PDF)
(But
then again, so do NiMH, NiZn, and high-drain Alkalines, without
most of the downsides of lithium.)
Energizer says the Advanced flavor lasts 4x longer in digital cameras than its Energizer Max alkaline, and Ultimate flavor lasts 8x as long.
Voltage drop. Lithiums drop their voltage
suddenly, like NiMH/NiCds. (source)
Don't confuse AA Lithiums with Lithium-Ion battery packs (like
the kind that come with laptops and many cell phones). Those
Lithium-Ion packs are rechargeable, but only when
they're installed in the device they're powering, or in a
special charger. Rechargeable Lithium-Ions are not available in
AAA-D sizes, though they're available in the 9V
size.
Shelf Life. At cool temperatures (68°F, 20°C), a
lithium battery will retain 90% of its charge for about 15
years. At higher temperatures the shelf life goes down,
but it's still pretty long (e.g., 90% after four years at
104°/40°C). (Energizer
PDF, p. 14)
Temperature. Lithiums work well in freezing temperatures, and are rated for a lower temperature than any other kind of battery (-40°F). Voltage and current flow remain good in cold temperatures. (source) I haven't yet found a source about capacity in cold temps, but I expect it to be good because lithiums are rated for cold-temperature use.
More: Energizer's tech specs (PDF)
Carbon Zinc (aka General Purpose, Heavy Duty, Zinc Chloride)
Pros:
- Really cheap
- Long shelf life
- An acceptable battery for low-drain devices like clocks,
radios, and remote controls
- Lowest capacity of any battery besides NiCd's
- An alkaline is usually better, even though it costs slightly
more
Summary. This is the original chemistry for household batteries, dating back to the 1800's! And it's still being used today, though of course better batteries have come along in the meantime. In fact, alkalines have all but replaced Carbon Zinc's. In any case where a Carbon Zinc would work, an alkaline will last even longer. The alkaline costs a little more, but it lasts 2-11x as long, making it the far better value. (Energizer)
If a package of batteries doesn't say what chemistry it is, it's almost certainly Carbon Zinc, Leclanché flavor. (See below.)
For years if you bought something and
batteries were included, they were almost certainly Carbon Zinc,
because that was the cheapest batteries that the manufacturer
could get away with. But alkalines aren't really that much
more expensive, and some manufacturers don't wish to appear
cheap, so alkalines are being bundled with new devices more and
more.
Naming. Some sources call these Zinc Carbon instead of Carbon Zinc, but I'll use the latter. And I'll abbreviate to CZ for short.
There are two flavors of CZ batteries:
- GENERAL PURPOSE. These are the original CZ, using the Leclanché method.
- (SUPER) HEAVY DUTY. These use the newer method, Zinc
Chloride, which offers 2-3x the capacity of Leclanché
batteries, better performance at lower temperatures, and are
less prone to leaking. They were "Heavy Duty" compared
to Leclanché batteries when they were introduced decades ago,
but compared to modern batteries, "Heavy Duty" are really
"Super Extremely Light Duty".
Nickel Oxyhydroxide (NiOx) discontinued
Pros:
- Exceptionally powerful (think brighter flashlights)
- Work great in high-drain devices
- Slow self-discharge rate (i.e., long shelf-life)
- No longer being made
- Can't be recharged (at least not normally)
- High voltage of 1.7 can burn out lights and some sensitive electronics
- Not available in C & D sizes, even when they were
available
Summary. After forty years, in 2005 NiOx became
the first new non-rechargeable battery format to challenge
alkalines. But they were too little, too late, since in
most cases rechargeable batteries like NiMH or NiZn provide
adequate power and are cheaper in the long run.
Manufacturers. Panasonic was first out of the gate
in 2005 with their Oxyride brand (2004 in Japan), but quickly
discontinued them circa 2009. Duracell followed with the
Duracell Ultra PowerPix, but discontinued theirs circa 2011.
Performance. NiOx batteries last more than twice as long
as standard alkalines in digital cameras, and around 1.5x as
long as high-drain alkalines like Duracell Ultra. But they
have worse performance in low-drain devices.
NiOx batteries put out 1.7 volts, higher than the 1.5 from an alkaline, and way higher than the 1.2 volts from an NiMH. The extra voltage should be safe for most devices, though I expect light bulbs may burn out a bit quicker. One bulb burned out in PC Magazane's test.
J.
Harper's independent tests show that Oxyrides are better
in freezing temperatures than alkalines. (Alkalines have
the worst performance at subfreezing temperatures.)
Charging. NiOx batteries can't normally be recharged. One person claims to be able to recharge oxyrides, but only with a slow charger, and only for six cycles. That's not nearly as much as an NiMH, which can be recharged hundreds of times.
More on Oxyride batteries:
- Oxyride
review in the New York Times, David Pogue
- Oxyride battery tests. Shows that oxyrides last much longer in cold temperatures vs. alkalines. They underperformed alkalines at normal temperatures, but that's because of the nature of the testa constant drain. In more typical use, oxyrides will beat alkalines.
- Wikipedia
on
Nickel Oxyhydroxide batteries
©1999-2021 by Michael Bluejay I have tried to verify all the information on this page, but I ain't responsible for no errors or omissions, bucko. Use at your own risk. Contact

Everything you wanna know. Shows you exactly how much you can save. (Visit now...)

Everything you wanna know. Shows you exactly how much you can save. (Visit now...)
|
Michael Bluejay’s
|
Contact • MBJ's home page |




