Getting the longest runtime & cycle life
from your batteries
"Runtime" is how long a battery can power a
device. It's basically the same as "capacity".
"Cycle Life" is how many times a battery can be recharged before it
won't charge any more, or its capacity has decreased too much.
Avoid alkalines for high-drain devices or for
cold-temperature use
Regular alkaline batteries are terrible in
high-drain devices (like cameras and motorized toys), because they
can't pump the power out fast enough. Rechargeable batteries will
actually outperform alkalines in this application. Yes,
there are special high-drain alkaline versions available, but they cost
more, and you're better off with NiMH which give great performance and
which can be recharged hundreds of times, so you can stop buying
batteries.
Ditto for use in cold temperatures, like bicycle lights in the
winter. Alkalines discharge fast when it's cold, but NiMH's are
much more robust.
For high-drain and cold-temperature use, I like eneloop
XX.
Charging strategies to maximize capacity and cycle life
Use a smart charger
Overcharging batteries hurts cycle life. Use a smart charger which gives your cells only as
much juice as they need.
Charge slow rather than fast
The faster you charge you cells, the lower the cycle
life. Four-five hours for a charge (regardless of battery size)
is probably a good compromise between convenience and preserving cycle
life. I couldn't dig up really excellent figures, but here's a forum thread with some independent testing.
Recharge sooner rather than later
You'll maximize recharge cycles of rechargeable batteries
by charging them before they're fully depleted. You don't have to
obsess about putting them in the charger after each time you use them,
but getting them in when they're down to only 1.1V instead of 1.0V
should give you a lot more cycle life. That's true of all
batteries, but it's especially true of NiZN and rechargeable alkaline.
Remove the batteries when they're done charging
Most chargers give a "trickle" charge after charging is
finished. Some chargers give too much of a trickle, and even
those which give a reasonable trickle can still overcharge if the
batteries are left in the charger too long. A good compromise
between convenience and not overcharging is to leave the batteries in
the charger no more than a day after being charged.
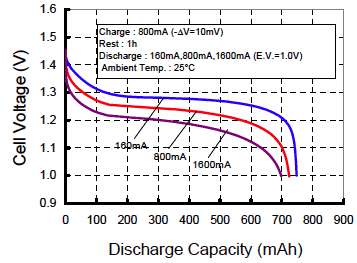
Realize that at faster discharge rates, you get less than
the rated
capacity
Capacity suffers a little bit when you use NiMH
batteries in a high drain device vs. one that gently sips the juice
slowly. The Sanyo chart at right suggests a penalty of about
7% when discharging very fast vs. slow. Here's another chart on Candlepower Forums.
I suspect that battery manufacturers test their batteries
with a slow discharge rate so they can claim a higher capacity, but
I haven't been able to find manufacturer's test methods, despite a lot
of searching for them. StefanV says the manufacturers use a 400 mA
discharge rate for AA's, but doesn't cite a source.
How temperature relates to battery life
Heat
kills batteries
- Under hot conditions, all batteries have a shorter
shelf
life, and a lower capacity when in use. For example:
- Over 4 years, an alkaline loses only ~1% of its
capacity
at 0°C/32°F, 11% at 20°C/68°F, and 25% at 40°C/104°F. (Energizer, PDF, p. 12)
- Over 15 years, a lithium will lose only 10% of its
capacity at 68°F (20°C), but 60% when stored at 104°F (40°C). (Energizer, PDF, p. 14)
Refrigerating batteries doesn't really help
Refrigerating batteries make sense only if
you're in a hot climate without air conditioning. (But even
then, manufacturers don't recommend that you put little chemical
factories near your food.) Here are
the details.
Alkalines. A long time ago the myth
started that putting alkalines in the fridge or freezer would help them
hold their charge. But alkalines already have great shelf life
anyway, so there's really no point. That is, alkalines already
hold
their charge properly for long periods of time (around 80% after 5-7
years). In 2000 Consumer
Reports compared 432 Duracell AA,
C, and D batteries in a refrigerator with some at room temperature.
After 2.5 years, refrigerated AA's had kept their charge perfectly, but
the unrefrigerated batteries lost only <4% of their charge. The
unrefrigerated C's & D's lost only 10%. (CR has since removed the
report from their site.) Refrigerating alkalines makes sense only
in very hot
climates without air conditioning. (They lose only ~5%/year at
85°F, but 25%/year at
100°F.) (Green Batteries) Though again, be careful
before putting chemical factories near your food.
NiMH. While the idea of refrigerating alkalines
was always an urban legend, it actually does work well for NiMH, but
there's not much point because you can simply use the low
self-discharge flavor of NiMH's in the first place.
By freezing NiMH's you can cut the first-month self-discharge from 20%
down to 10%. (Source: Green Batteries)
Use it or lose it?
Battery University says that NiMH's lose some of
their capacity permanently if not used for
long periods of time. (Battery
University)
You can also see our charging
tips.
(Google picks the ads, not me.)
Last update: January 2013
|